OVERVIEW
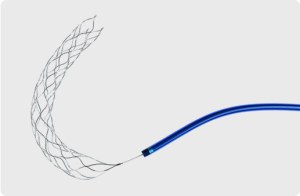
Thrombite™ Clot Retriever Device
HELICAL OPEN-SIDE STRUCTURE
HIGHER ACUTE RECANALIZATION RATE
The Thrombite™ endovascular clot retrieval , featuring S-shaped helical open-side structure, is designed for more efficient clot removal and optimum revascularization in a wide range of vessels.
PRODUCT FEATURESHELICAL OPEN-SIDE STRUCTURE
enables Thrombite™ clot retriever to efficiently entwine and clamp the clot
assures clot retention for confident removal and revascularization
OVERLAPPED STENT AT THE HELICAL OPEN-SIDE STRUCTURE
increases contact surface with thrombus and maximizes clot integration
EXCELLENT FLEXIBILITY AND VESSEL WALL APPOSITION
optimizes clot retention during smooth navigation through the tortuous anatomies
Radiopaque markers on the distal end of the device increase device visibility
3 markers for the sizes with 3.0 and 4.0mm diameter
4 markers for the sizes with 5.0 and 6.0mm diameter
Advanced stent surface treatment process
RADIAL FORCE
High radial force is designed for clot integration at the initial stage of stent expansion
Low radial force at nominal stent diameter ensures atraumatic retrieval
RADIAL FORCE
How a Stent Retriever Procedure Is Performed
Stent retriever procedure is generally performed in patients with the indications of acute cerebral infarction within six hours. After femoral artery puncture, the catheter is inserted after sheath puncture, and DSA is performed along the catheter to the brain to find the position of blocked vascular. Make the stent retriever reached the position and passing through the clot. Then release to capture the clot. After that, the stent retrieval device will be extracted along with the clot and finally leave your body.
ORDERING INFORMATION OF CLOT RETRIEVAL DEVICE
STENT RETRIEVAL DEVICE STRUCTURE
Thrombite™ Product code Diameter D(mm) Recommended Vessel Diameter(mm) Recommende Microcatheter ID (inch) Working Length A(mm) Stent Length B(mm) System Length(mm)
- Max.
3MM CRD-3-15 3 1.5 3 0.21 15 27 1900
CRD-3-20 3 1.5 3 0.21 20 32 1900
CRD-3-25 3 1.5 3 0.21 25 37 1900
CRD-3-30 3 1.5 3 0.21 30 42 1900
4MM CRD-4-15 4 2 4 0.21 15 27 1900
CRD-4-20 4 2 4 0.21 20 32 1900
CRD-4-25 4 2 4 0.21 25 37 1900
CRD-4-30 4 2 4 0.21 30 42 1900
5MM CRD-5-15 5 2.5 5 0.027 15 27 1900
CRD-5-20 5 2.5 5 0.027 20 32 1900
CRD-5-25 5 2.5 5 0.027 25 37 1900
CRD-5-30 5 2.5 5 0.027 30 42 1900
6MM CRD-6-15 6 3 5.5 0.027 15 27 1900
CRD-6-20 6 3 5.5 0.027 20 32 1900
CRD-6-25 6 3 5.5 0.027 25 37 1900
CRD-6-30 6 3 5.5 0.027 30 42 1900
INDICATIONS
Thrombite™ Clot Retriever Device
The Thrombite™ clot retrieval device is intended to restore blood flow by removing thrombus from a large intracranial vessel in patients experiencing ischemic stroke symptom onset.Zylox Tonbridge Medical highlights the corporate social responsibility and mission, and boosts the innovation and technological development by taking active part in the combination of scientific innovation with medical engineering.
Ubicación : No. 270, Shuyun Road, Cangqian Street, Hangzhou, Zhejiang Province, P.R.China, 311121 Zyloxtb,
Persona a contactar : .com Zyloxtb, 0571 88610082